Adapting to the Evolving European Clinical Trial Regulatory Scenario: An Overview of the Current State of the European Clinical Trials Regulation and Clinical Trials Information System - ACRP

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging
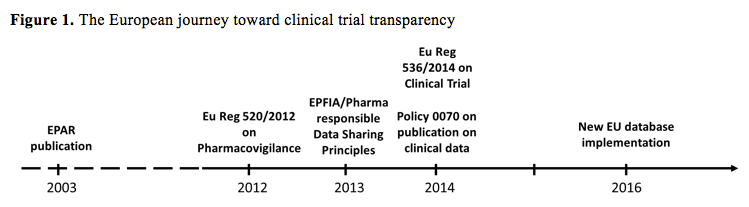
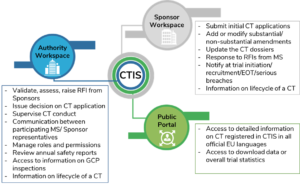
The implementation of the Clinical Trial Regulation (Regulation (EU) No 536/ 2014): where do we stand?

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging

EMA Webinar for SMEs and Academia on the Clinical Trials Regulation and the Clinical Trials Information System | ERICA

European Clinical Trials Regulation No. 536/2014: Hutchinson, Prof. D.: 9781908278500: Books: Amazon.com

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging